"Uncover the truth behind the groundbreaking discovery of the causes of the world's biggest problems. 'The Root Causes' will change how you view the world!"
Nutritional Influences on Autism: The Significance of Breastfeeding and Infant Formula
This blog examines the critical role of folate in early synaptic development and neuroplasticity, emphasizing how cow’s milk–based infant formulas may reduce folate availability through molecular mimicry, triggering folate receptor alpha autoantibodies (FRAAs) that block folate transport to the brain. It also highlights the protective effects of breastfeeding and the potential amplifying role of acetaminophen on neurodevelopmental vulnerability. ✅ For informational purposes only; not medical advice. Suggested Tags (unchanged): #Autism #ASD #Breastfeeding #InfantFormula #CowMilk #Folate #FRAA #SynapticDevelopment #Neurodevelopment #Acetaminophen #ChildHealth #EarlyNutrition #Microbiome #CognitiveDevelopment #ImmuneHealth #Parenting #MedicalResearch
Glenn Rosaroso Vale, MT(AMT), MS(IT), MBA
9/25/20255 min read
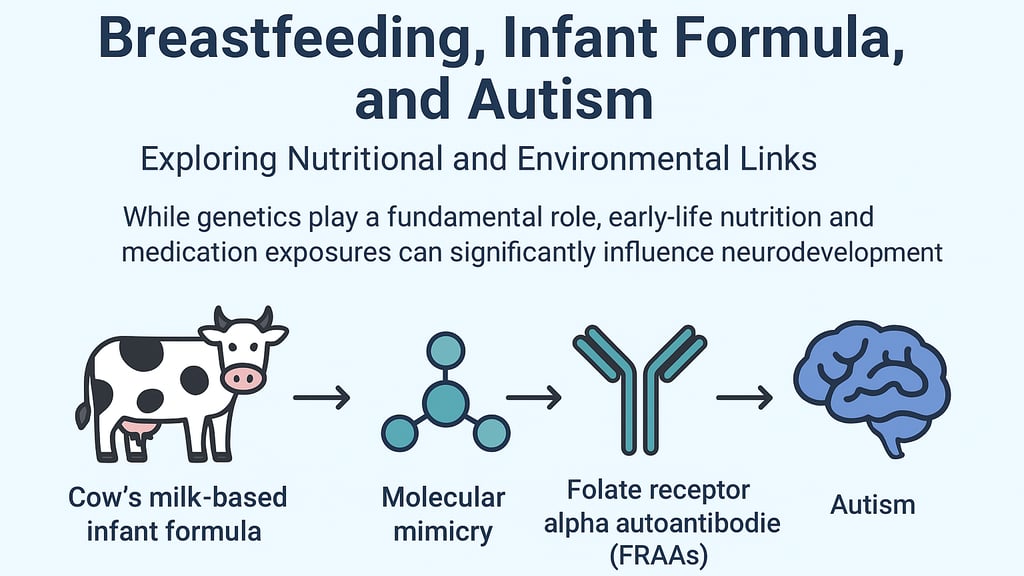
Breastfeeding, Infant Formula, and Autism: Exploring Nutritional and Environmental Links
Introduction
Autism spectrum disorder (ASD) continues to rise in prevalence, with current estimates suggesting that 1 in 31 U.S. children are affected (Centers for Disease Control and Prevention [CDC], 2025). While genetics play a central role, researchers are increasingly investigating nutritional and environmental factors that may influence early neurodevelopment. Among these, breastfeeding, cow’s milk–based infant formulas, and the use of acetaminophen (Tylenol) have drawn attention as potential contributors or amplifiers of autism risk.
The Role of Breastfeeding in Early Neurodevelopment
Breastfeeding provides far more than calories. Human breast milk is rich in:
Long-chain polyunsaturated fatty acids (DHA, ARA): essential for synapse formation, neuronal growth, and cognitive function (Lauritzen et al., 2016).
Human milk oligosaccharides (HMOs): nourish beneficial gut bacteria, supporting the microbiome and immune system (Bode, 2015).
Immunoglobulins and growth factors: protect against inflammation and infection (Ballard & Morrow, 2013).
Active folates and B vitamins: crucial for methylation reactions, neurotransmitter synthesis, and synaptic plasticity (Ramaekers et al., 2007).
Several studies indicate that longer breastfeeding duration is associated with lower autism risk and improved cognitive outcomes (Al-Farsi et al., 2012; Tseng et al., 2019). Breastfeeding provides a protective biochemical environment that buffers the developing brain against oxidative stress, immune dysregulation, and gut-derived inflammation, all of which are implicated in autism.
Cow’s Milk–Based Infant Formula: Casein, Molecular Mimicry, and FRAAs
Infants who are not breastfed often rely on cow’s milk–based formulas, which contain bovine casein proteins. In some genetically susceptible children, these proteins may disrupt normal neurodevelopment through molecular mimicry and immune-mediated mechanisms:
Casein-derived peptides (casomorphins):
When incompletely digested, casein can release opioid-like peptides capable of crossing the gut barrier, affecting neurotransmission and altering behavior, pain perception, and social interaction (Cass et al., 2008).Molecular mimicry and folate receptor alpha autoantibodies (FRAAs):
Certain bovine casein peptides closely resemble human folate receptor alpha (FRα) epitopes. The immune system may mistakenly produce autoantibodies against FRα after exposure to these proteins (Ramaekers et al., 2008; Frye et al., 2013).Mechanism: FRAAs bind to FRα in the choroid plexus, blocking transport of folate across the blood-brain barrier.
Consequence: Reduced cerebral folate levels lead to impaired methylation, defective synaptic development, and altered neurotransmission, which are critical processes in early brain development and plasticity (Ramaekers et al., 2007).
Inflammatory response:
Cow’s milk proteins can also trigger gut inflammation and systemic immune activation, particularly in predisposed infants (Waserman & Watson, 2011). This immune activation synergizes with FRAA-mediated folate blockade to further compromise synaptic maturation and neural circuitry.
Infant Formula, the Microbiome, and Neurological Health
Unlike breast milk, infant formula lacks live probiotics and HMOs, which feed beneficial gut microbes. Consequences include:
Reduced gut microbial diversity.
Increased intestinal permeability ("leaky gut").
Amplified neuroimmune interactions, potentially exacerbating autism risk (Hsiao, 2014).
The combination of formula feeding, molecular mimicry, and immune activation creates an environment where folate-dependent synaptic development is disrupted, highlighting a mechanistic link between early nutrition and autism pathophysiology.
Acetaminophen (Tylenol) as an Amplifier
Acetaminophen exposure in infancy may act as a biochemical amplifier of neurodevelopmental vulnerability:
Glutathione depletion: Reduces antioxidant defenses, increasing oxidative stress (James et al., 2005).
Neuroinflammation: When combined with immune activation from cow’s milk proteins, acetaminophen may worsen synaptic vulnerability (Bittker & Bell, 2018; Schultz et al., 2019).
Thus, infants exposed to cow’s milk–based formulas and acetaminophen may experience a "perfect storm," where oxidative stress, immune-mediated folate blockade, and impaired synaptic development converge to elevate autism risk.
Conclusion
Genetics are fundamental, but early-life nutrition and medication exposures can significantly influence neurodevelopment:
Breastfeeding supplies protective compounds that support synaptic formation and reduce inflammation.
Cow’s milk–based formula may, through molecular mimicry and FRAA formation, impair folate transport and disrupt synapse development.
Acetaminophen use may further amplify these risks by weakening antioxidant and detoxification pathways.
Supporting breastfeeding, considering hypoallergenic formulas for at-risk infants, and limiting unnecessary acetaminophen may help protect developing synapses and reduce neurodevelopmental vulnerability.
✅ Disclaimer: This blog is for informational purposes only and is not medical advice. Parents should consult qualified healthcare professionals before making feeding or medication decisions.
References
Al-Farsi, Y. M., Waly, M. I., Deth, R. C., Al-Sharbati, M. M., Al-Shafaee, M. S., Al-Farsi, O., ... & Al-Adawi, S. (2012). Low folate and vitamin B12 status and potential metabolic effects among children with autism. Nutrition, 29(2), 537–541. https://doi.org/10.1016/j.nut.2012.08.002
Ballard, O., & Morrow, A. L. (2013). Human milk composition: nutrients and bioactive factors. Pediatric Clinics of North America, 60(1), 49–74. https://doi.org/10.1016/j.pcl.2012.10.002
Bittker, S. S., & Bell, K. R. (2018). Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and autism: An epidemiological study. Neuropsychiatric Disease and Treatment, 14, 1399–1414. https://doi.org/10.2147/NDT.S157741
Bode, L. (2015). The functional biology of human milk oligosaccharides. Annual Review of Nutrition, 32, 55–75. https://doi.org/10.1146/annurev-nutr-071212-141307
Cass, H., Gringras, P., March, J., McKendrick, I., O’Hare, A. E., Owen, L., & Pollin, C. (2008). Absence of urinary opioid peptides in children with autism. Archives of Disease in Childhood, 93(9), 745–750. https://doi.org/10.1136/adc.2007.128892
Centers for Disease Control and Prevention. (2025). Autism spectrum disorder (ASD): Data and statistics. https://www.cdc.gov/autism
Frye, R. E., Sequeira, J. M., Quadros, E. V., James, S. J., & Rossignol, D. A. (2013). Cerebral folate receptor autoantibodies in autism spectrum disorder. Molecular Psychiatry, 18(3), 369–381. https://doi.org/10.1038/mp.2011.175
Hsiao, E. Y. (2014). Immune dysregulation in autism spectrum disorder. International Review of Neurobiology, 113, 269–302. https://doi.org/10.1016/B978-0-12-418700-9.00008-2
James, S. J., Melnyk, S., Jernigan, S., Hubanks, A., Rose, S., & Gaylor, D. W. (2005). Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. The American Journal of Clinical Nutrition, 80(6), 1611–1617. https://doi.org/10.1093/ajcn/80.6.1611
Lauritzen, L., Brambilla, P., Mazzocchi, A., Harsløf, L. B. S., Ciappolino, V., & Agostoni, C. (2016). DHA effects in brain development and function. Nutrients, 8(1), 6. https://doi.org/10.3390/nu8010006
Ramaekers, V. T., Blau, N., & Quadros, E. V. (2007). Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics, 38(6), 276–281. https://doi.org/10.1055/s-2007-993145
Ramaekers, V. T., Rothenberg, S. P., Sequeira, J. M., Opladen, T., Blau, N., Quadros, E. V., & Selhub, J. (2008). Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. New England Journal of Medicine, 352(19), 1985–1991. https://doi.org/10.1056/NEJMoa043160
Schultz, S. T., Klonoff-Cohen, H. S., Wingard, D. L., Akshoomoff, N. A., Macera, C. A., Ji, M., & Bacher, C. (2019). Acetaminophen use, measles-mumps-rubella vaccination, and autistic disorder: The results of a parent survey. Autism, 12(3), 293–307. https://doi.org/10.1177/1362361308089516
Tseng, P. T., Cheng, Y. S., Yen, C. F., Chen, Y. W., Stubbs, B., Whiteley, P., ... & Carvalho, A. F. (2019). Maternal breastfeeding and autism spectrum disorder in children: A systematic review and meta-analysis. Nutrients, 11(8), 1–14. https://doi.org/10.3390/nu11081702
Waserman, S., & Watson, W. (2011). Food allergy. Allergy, Asthma & Clinical Immunology, 7(Suppl 1), S7. https://doi.org/10.1186/1710-1492-7-S1-S7
Health
Understanding illness to empower your well-being journey.
Wellness
Knowledge
info@rootcauseprevention.com
903-268-6664
© 2024. All rights reserved.
grfv@sbcgloal.net